Currently, our disclosed development pipeline is following.
<Pharmaceuticals>
| Development code, substance name |
Indication | Developed in | Stage | Remarks |
|---|---|---|---|---|
| SI-6603 Condoliase |
Lumbar disc herniation | USA | Application | Exclusive Worldwide License Agreement (excluding Japan) for SI-6603 with Ferring Pharmaceuticals (Switzerland) |
| Gel-One cross-linked hyaluronate |
knee and hip osteoarthritis |
Japan | Phase III | Co-development and Marketing Collaboration Agreement in Japan for Gel-One with Ono Pharmaceutical Co., Ltd. |
| SI-722 Chondroitin Sulfate- Steroid Conjugates |
Interstitial Cystitis and Bladder Pain Syndrome |
USA | Phase I/II |
<Medical Devices>
| Development code, substance name |
Description | Developed in | Stage | Remarks |
|---|---|---|---|---|
| SI-449 Cross-linked Chondroitin Sulfate |
Adhesion barrier | Japan | Application |
(As of August 20, 2025)
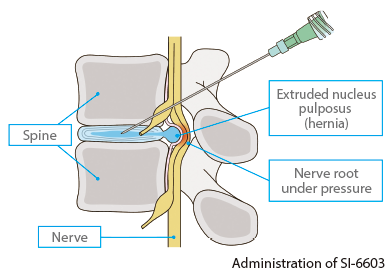
SI-6603, which contains condoliase as its active pharmaceutical ingredient, is a treatment for lumbar disc herniation directly injected into the intervertebral disc. It has the special characteristic of not requiring general anesthesia and being less invasive to patients than surgical treatment. Since a single injection is expected to improve the symptoms of lumbar disc herniation by reducing intervertebral disc pressure and relieving pressure on the nerve root, SI-6603 can contribute to improving patients’ quality of life as a new treatment option.
In Japan, marketing approval was obtained from the Ministry of Health, Labour and Welfare in March 2018, and SI-6603 was launched on August 1, 2018 as HERNICORE 1.25 units for intradiscal injection.
Subject enrollment for an additional Phase III clinical study being conducted in the U.S. since February 2018 was completed in March 2022. In May 2023, we obtained statistically significant topline results for the primary endpoint from an additional Phase III clinical study in the U.S. for SI-6603. In May 2024, Biologics License Application ("BLA") for SI-6603 has been accepted for filing by the U.S. Food and Drug Administration ("FDA").
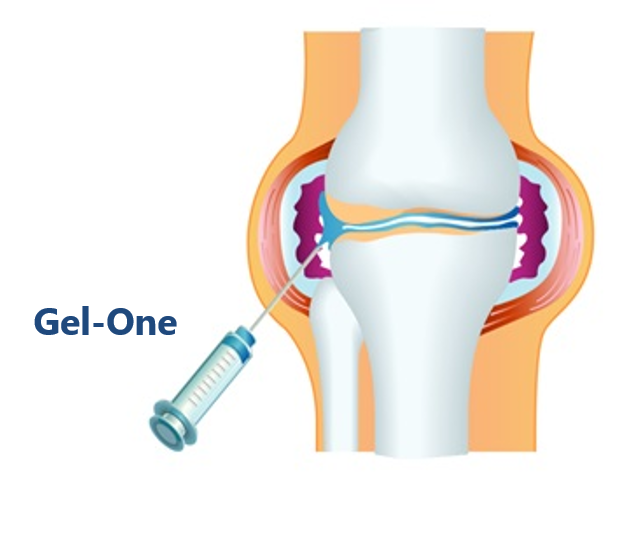
Gel-One is an intra-articular injection whose active ingredient is a cross-linked hyaluronate, utilizing Seikagaku’s unique cross-linking technology. Since Gel-One remains in the joint for a long period of time, a single injection of Gel-One is expected to provide long-term pain relief.
The product has been available in overseas markets since 2012 as Gel-One (U.S.) and HyLinkⓇ (Taiwan and Italy) indicated for osteoarthritis of the knee.
In Japan, three Phase III clinical studies are planned, a study for osteoarthritis of the knee, a study for osteoarthritis of the hip, and a long-term safety study for both joints. Administration of Gel-One to the first subject has started on February 2025.
Additionally, whereas the indication for Gel-One in overseas markets is the knee joint, in Japan, Seikagaku will concurrently conduct a study of Gel-One for osteoarthritis of the hip alongside the knee study with the aim of expanding the target patient population.
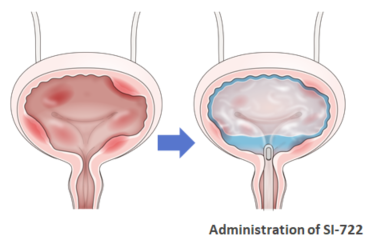
SI-722 is a novel chemical compound in which a steroid is conjugated with chondroitin sulfate using Seikagaku's proprietary glycosaminoglycan modification technology and drug delivery systems. SI-722 injected into the bladder is thought to demonstrate an improvement effect in symptoms such as of frequent urination and bladder pain, by releasing a steroid with an anti-inflammatory effect.
Seikagaku is considering the policy on future development based on data obtained in Phase I/II clinical studies.
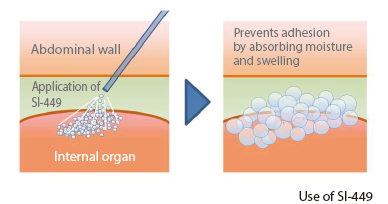
SI-449 is a powdered adhesion barrier whose main ingredient is cross-linked chondroitin sulfate developed using Seikagaku’s own glycosaminoglycan cross-linking technology. SI-449, which has the property of absorbing moisture and swelling, is expected to prevent or mitigate post-operative adhesion formation by forming a barrier between the surgical wound site and surrounding tissues after application. Development of this subject is progressing with an eye not only on domestic development, but also globally.
Subject enrollment for a pivotal study in the field of gastroenterological surgery was completed in September 2022. The study is being conducted to confirm efficacy (prevention of adhesion formation), safety, and usability in gastroenterological surgery. Also, subject enrollment for a pilot study in the field of gynecology aimed at expanding the scope of application of SI-449 was completed in May 2022. Following the observation periods of both studies, the Company submitted an application for marketing approval in August 2025.