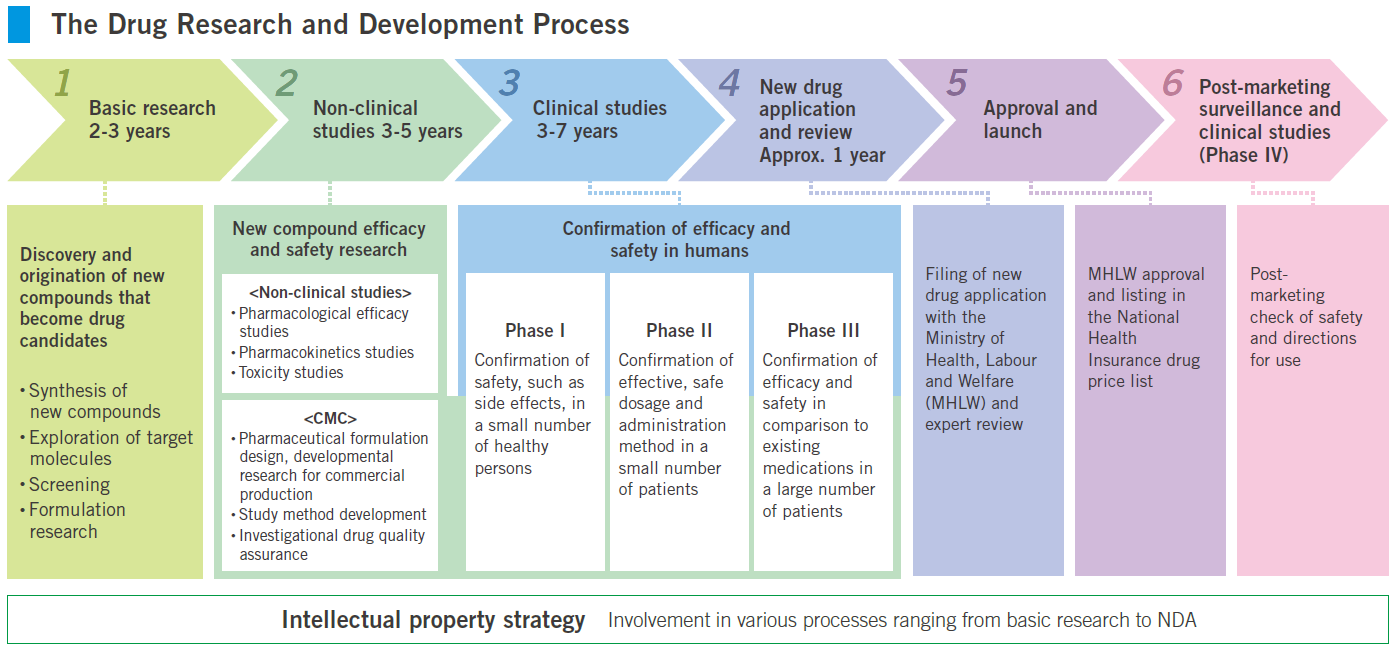
To create new drugs, it is necessary to conduct various studies to evaluate efficacy and safety. Clinical studies are conducted to confirm whether drug candidates are actually beneficial to humans, following completion of research processes such as basic research and non-clinical studies.
Clinical studies are ordinarily divided into three phases and conducted at medical institutions such as hospitals in conformance with rigorous standards after the consent of subjects (healthy persons or patients) has been obtained.
A Phase I clinical study, the initial phase, is ordinarily conducted for the main purpose of examining the pharmacokinetics (absorption, distribution, metabolism, and excretion) and safety (adverse events and side effects) of investigational drugs in a small number of healthy subjects. A Phase II clinical study examines efficacy, safety, and pharmacokinetics and confirms optimal dosage and usage in a small number of patients. A Phase III clinical study, the final phase, objectively verifies efficacy and safety in comparison to existing approved drugs or placebos in large numbers of patients.A Phase I clinical study, the initial phase, is ordinarily conducted for the main purpose of examining the pharmacokinetics (absorption, distribution, metabolism, and excretion) and safety (adverse events and side effects) of investigational drugs in a small number of healthy subjects. A Phase II clinical study examines efficacy, safety, and pharmacokinetics and confirms optimal dosage and usage in a small number of patients. A Phase III clinical study, the final phase, objectively verifies efficacy and safety in comparison to existing approved drugs or placebos in large numbers of patients.
Ordinarily, more than ten years is required from discovery of a candidate substance until its approval as a new drug. Within the long, difficult new drug development process, clinical development is considered to hold the key to whether an NDA can be filed.