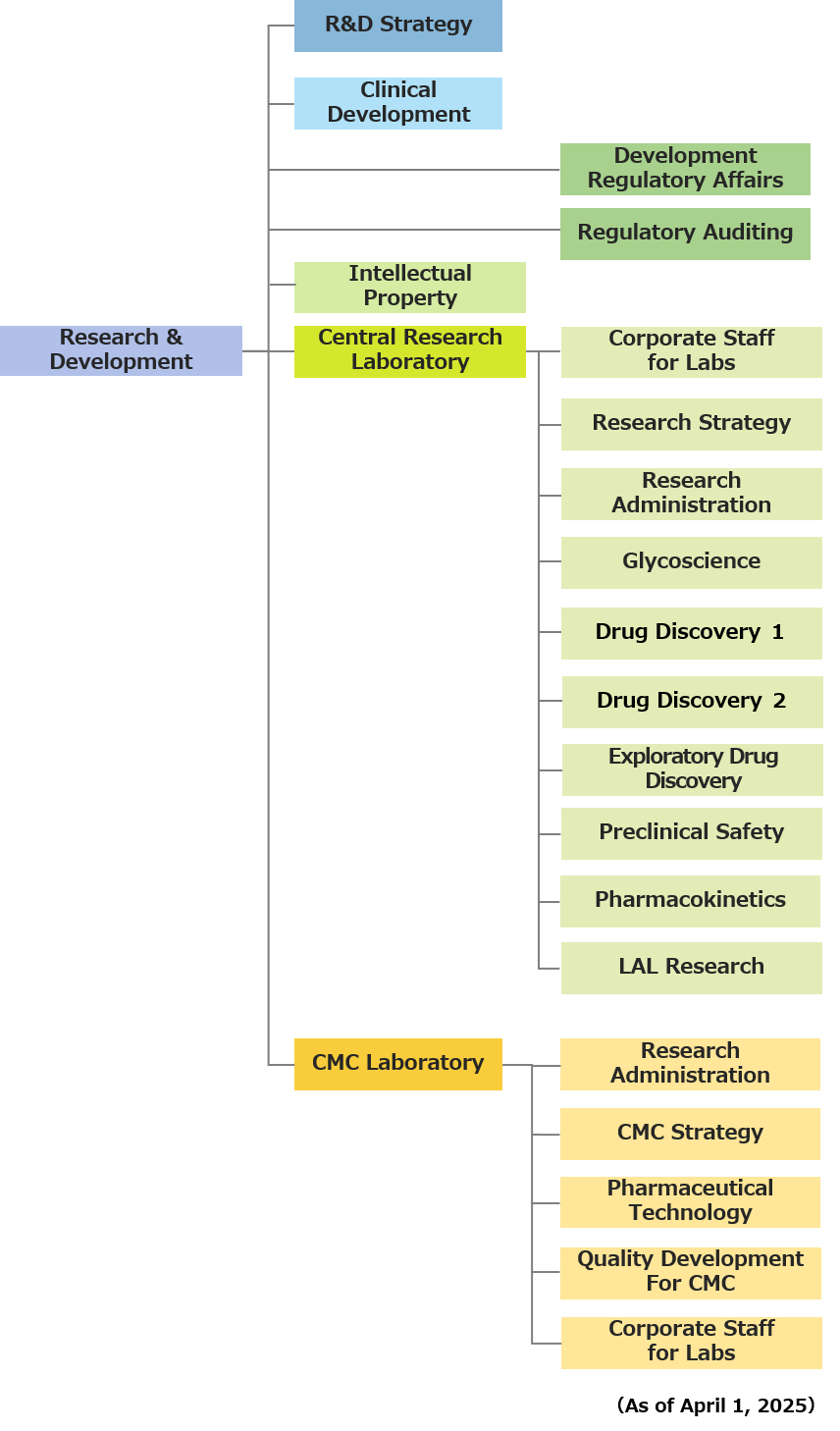
To ensure close coordination of the drug development process from its upstream to downstream, Seikagaku has put in place an organizational structure in which the departments involved in R&D are consolidated under the control of the Research & Development Division.
This integrated organization covers every R&D activity from clinical development to new drug application (NDA) and intellectual property strategy. In this structure, the Central Research Laboratory is in charge of exploring candidate substances and evaluating efficacy, safety, and pharmacokinetics, and the CMC* Laboratory is responsible for production of investigational drugs, design of manufacturing processes, and consideration of commercial production.
*CMC is an abbreviation for Chemistry, Manufacturing and Controls, which refers to the physicochemical properties and standards of active pharmaceutical ingredients (API) and formulations, their manufacturing processes, and quality control.
The Central Research Laboratory, Seikagaku’s drug discovery research center, cultivates the creativity of researchers in a fulfilling research environment, equipped with advanced facilities, and places importance on fostering a self-help culture.
Seikagaku contributes unique knowledge, technology, and expertise related to glycoscience to benefit drug discovery research, and actively collaborates with universities and companies in Japan and overseas to accelerate the search for ideas and development of new technologies. Through these efforts, we work to create original pharmaceuticals and medical devices on the basis of specialized technologies and creative ideas.

- 拡大
- Central Research Laboratory and CMC Laboratory
<Overview of Research Units>
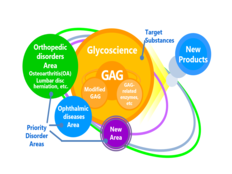
- Glycoscience: Exploration of GAG (glycosaminoglycans) and related compounds as pharmaceutical candidate substances
- Drug Discovery: Synthesis of new candidate substances with GAG as the basis for research, evaluation of their efficacy and function, and research on their actions and mechanisms
- LAL Research: Development of manufacturing technologies for reagents and diagnostics based on exploratory research of new technologies
The CMC Laboratory produces investigational drugs, designs manufacturing processes, engages in quality development, and examines commercial production of products under development created by the Central Research Laboratory.
By engaging in development from the R&D stage in collaboration with the Production Division, the CMC Laboratory aims to ensure the stable supply of high-quality pharmaceuticals and medical devices that comply with regulations in Japan, the United States, and Europe and to increase the speed of new drug development under a system integrated from research to production.
<Overview of Research Units>
- Pharmaceutical Technology: Design of active pharmaceutical ingredients, pharmaceutical formulations, packaging, and manufacturing processes for candidate substances and consideration of commercial production
- Quality Development for CMC: Research of physicochemical properties, development of testing methods for quality evaluation, and quality assurance of investigational drugs
Seikagaku conducts various clinical studies in Japan and the U.S. in cooperation and collaboration with medical experts, medical institutions, and external contract research organizations (CROs) and site management organizations (SMOs). The Clinical Development Department is responsible for the creating the integrated development plan (protocols); monitoring of clinical studies; planning and execution of enrollment acceleration; and data management and analysis of study results. It also communicates with the regulatory authorities in various countries and develops dossiers necessary at the time of NDA filing.
In developing protocols, the Clinical Development Department closely communicates with medical monitors and regulatory authorities, identifies requirements for NDA approval and finalizes the study design. In monitoring of clinical studies, the Department works through medical institutions to ensure the quality of studies by confirming whether they are being conducted in conformance with Good Clinical Practice (GCP) and regulatory requirements by ascertaining the condition of subjects and reviewing study data.
The R&D Strategy Department is in charge of planning basic strategies for R&D and the progress management for each R&D subject. The department also gathers information related to cross-divisional R&D and comprehensively understands and manages the progress of research subjects at each stage of development to facilitate the promotion of R&D activities.
In addition to application approval for newly developed products and the review with regulatory authorities regarding pharmaceutical affairs, the Development Regulatory Affairs Unit deals with the investigation of application requirements and support for creating submissions that receive early approval in countries where we aim to obtain manufacturing/marketing approval.