Seikagaku’s mission is to provide patients with a continuous supply of safe, benecial, high-quality pharmaceuticals and medical devices. We have constructed corporate quality assurance and compliance systems in accordance with laws, regulations, and standards.
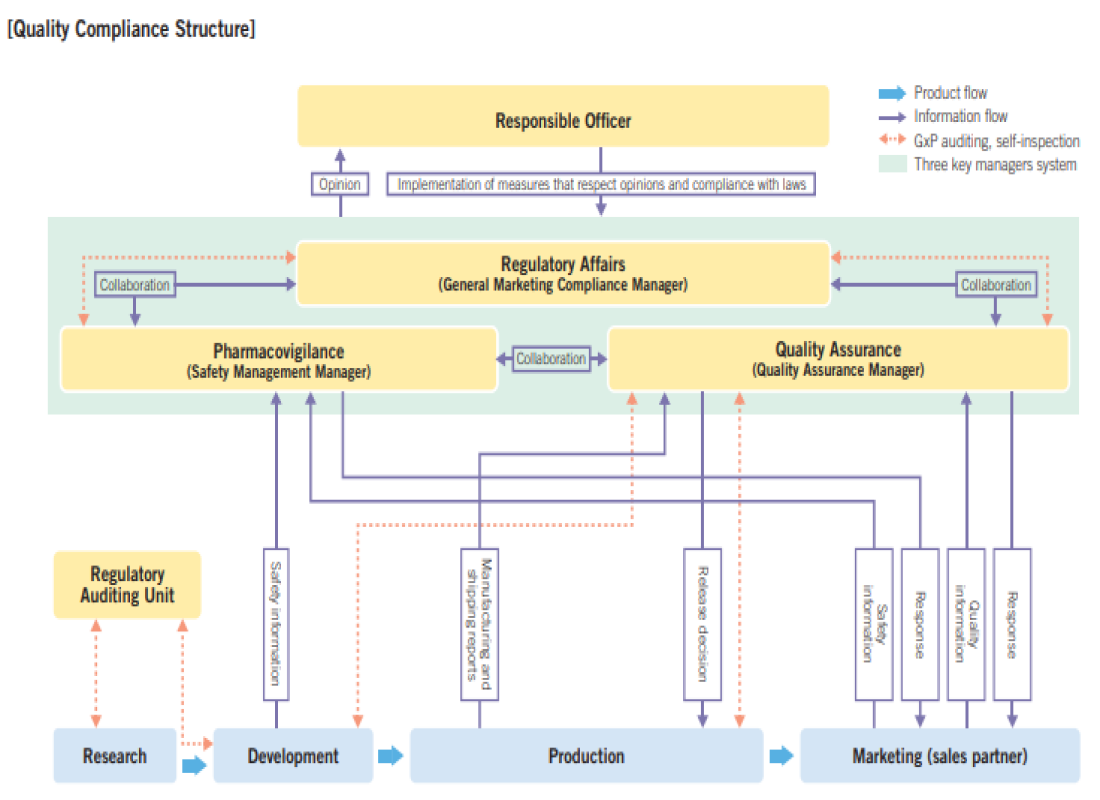
Seikagaku makes maximum effort to ensure quality at every stage, from R&D to post-marketing, by complying with the pharmaceutical laws and regulations of overseas countries, including a collection of regulations and guidelines called GxP*. In Japan, as a marketing authorization holder, we have developed a system with three key roles (general marketing compliance officer, quality assurance supervisor, and safety management supervisor) and implement appropriate quality management and pharmacovigilance operations in accordance with the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices (“PMD Act”).
To continue to reliably provide pharmaceuticals and medical devices required by patients around the world, we will strive to maintain and enhance quality assurance and compliance systems in accordance with global standards.
*GxP is an abbreviation for Good XXX Practice, a collective term for standards established to ensure the efficacy, safety, and quality of pharmaceuticals and medical devices from the R&D stage to post-marketing.
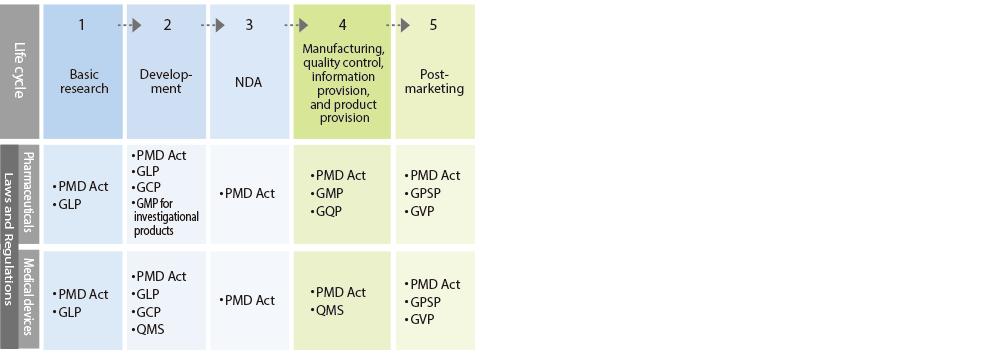
- PMD (Pharmaceutical and Medical Device) Act
Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices - GLP:Good Laboratory Practice
Standards for conducting non-clinical studies on safety - GCP:Good Clinical Practice
Standards for conducting clinical studies - GMP:Good Manufacturing Practice
Standards for manufacturing control and quality control in manufacturing - GVP:Good Vigilance Practice
Standards for post-marketing safety management of drugs, quasi-drugs,
cosmetics and medical devices and regenerative medicine products - GQP:Good Quality Practice
Standards for quality control of products - GPSP:Good Post-marketing Study Practice
Standards for conducting post-marketing surveys and studies on drugs - QMS:Quality Management System
Ordinance on Standards for Manufacturing Control and Quality Control of Medical Devices and In Vitro Diagnostic Reagents
To provide a stable supply of high-quality pharmaceuticals and medical devices, in accordance with our Quality Policy, we have developed a world-class quality management system. At the development stage, we ensure reliability under Good Laboratory Practice (GLP) and Good Clinical Practice (GCP) standards. To ensure compliance with laws and regulations and maintenance of quality assurance systems through the post-marketing stage, each year we systematically conduct self-inspections and internal audits to confirm the status of operation of the quality management system and promptly take corrective and preventive actions as necessary.
Seikagaku has obtained ISO 13485 certification for the development, manufacture, and distribution of sodium hyaluronate-based viscoelastic products for the treatment of osteoarthritis of the knee and periarthritis of the shoulder. We strictly maintain and control quality at all stages from product design and development to post-marketing in conformance with these manufacturing control and quality assurance systems.
ISO 13485 is an international standard for quality management systems established by the International Organization for Standardization (ISO) that prescribes requirements concerning the design, development, and manufacturing of medical devices. In Japan, ISO 13485 has been adopted as an ordinance on standards for manufacturing control and quality control of medical devices and in vitro diagnostics.
Sometimes side effects not observed in the development stage come to light after the launch of a new pharmaceutical product. In accordance with Good Vigilance Practice (GVP) standards, Seikagaku conducts post-marketing pharmacovigilance activities involving promptly and appropriately collecting, evaluating, and sharing feedback information on the side effects of pharmaceuticals prescribed at medical facilities. Through these activities, we prevent the expansion of side effects and promote safety assurance and appropriate use of new drugs.
Seikagaku has established the Medical Affairs Unit, which engages in activities to provide current scientific knowledge to external professionals independently from the marketing division. As scientific experts with sufficient ethical perspective, the unit contributes to medical progress by creating and disseminating medical evidence relating to disease information and products in the fields in which Seikagaku focuses, such as orthopedic disorders and ophthalmic diseases.